|
ZIRCONIUM HYDRIDE |
|
PRODUCT
IDENTIFICATION
|
| CAS
NO. |
7704-99-6
(ZrH4), 13940-37-9
(ZrH2)
|
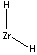
|
| EINECS
NO. |
231-727-3 |
| FORMULA |
ZrH2
|
| MOL
WT. |
93.23 |
|
H.S.
CODE
|
|
| TOXICITY |
|
| SYNONYMS |
Dihydrure de
zirconium (French); |
| Zirconium Dihydride (German); Dihidruro de Circonio (Spanish); |
|
CLASSIFICATION
|
|
|
PHYSICAL AND CHEMICAL PROPERTIES
|
| PHYSICAL
STATE |
gray-black powder
|
| MELTING POINT |
|
| BOILING
POINT |
|
| SPECIFIC GRAVITY |
|
| SOLUBILITY
IN WATER |
Insoluble
|
| pH |
|
| VAPOR DENSITY |
|
|
NFPA RATINGS
|
|
| AUTOIGNITION |
|
| FLASH
POINT |
None flammable |
| STABILITY |
Stable under normal
conditions |
|
GENERAL
DESCRIPTION OF ZIRCONIUM & ITS COMPOUNDS |
Zirconium is a metallic transition element in Group IVb of periodic table; symbol
Zr, hard,
malleable, ductile, lustrous, silvery gray; atomic number 40; atomic mass
91.224; melting point ca 1,852°C; boiling point ca 4,377°C; specific gravity
6.56 g/cm3; valence +2,+3 or +4; electronic config. [Kr]4d25s2. It is light but
Its hardness is similar to copper. It has a hexagonal close-packed crystalline
structure resembles titanium. It is insoluble in water, soluble in hot or
concentrated acids, flammable as powder. Zirconium is mainly (almost 90% among
whole zirconium production) used in nuclear reactors due to its property of low
absorption for thermal neutrons, in addition to its extremely resistance to heat
and corrosion. It is applied in tubes for cladding uranium oxide fuel. Zirconium
is a fairly abundant element and the chief ore is zircon (neosilicate mineral
occurring in tetragonal prisms, ZrSiO4, also known as hyacinth; jacinth;
zirconite) which contains Zirconium and hafnium at a ratio of about 50 : 1.
Hafnium which absorbs neutrons readily should be removed for the usage of
zirconium in nuclear reactors. The other ores are baddeleyite (the oxide),
monazite, ilmenite, and rutile. Zirconium is usually alloyed with other metals
to make corrosion-resistant alloys. The chief comsumption in chemical industry
field is for piping against corrosion, in ceramic, refractory compounds,
pyrotechnics, welding fluxes, and explosives. Zirconium compounds are also used in
TiO2 pigment coating and leather
tanning. Zirconium forms a number of
compounds as below:
- Zirconia (also
known as zirconium oxide or as zirconic anhydride, ZrO2 ): toxic,
heavy white powder that is insoluble in water, soluble in mineral acids; melts
at 2700°C; used in ceramic glazes, special glasses, as an abrasive, a refractory material, and a component of
acid- and alkali-resistant glasses and of ceramics employed in fuel cells and medicine, and to make
piezoelectric crystals.
- Zirconium
Acetate (ZrC2H4O2, CAS RN: 7585-20-8) used as a flame retardants for textiles.
- Zirconium
Neodecanoate (ZrC40H76O8,
CAS RN: 39049-04-2)
- Zirconium Acetylacetonate
((C5H7O2)4Zr, CAS RN: 17501-44-9)
- Zirconium Boride
(ZrB2,
CAS RN: 12045-64-6): toxic, gray, hard powder;
melting at 3000°C; used as an aerospace
refractory, in cutting tools, and to protect thermocouple tubes.
- Zirconium Butoxide (C16H36O4,
CAS RN: 1071-76-7)
- Zirconium Carbide (ZrC,
CAS RN: 12070-14-3): Hard, gray crystals or powder;
soluble in water and acids; ignites spontaneously in air;
melting at 3400°C, boiling at 5100°C; used as an abrasive, refractory, and metal
cladding, and in cermets, incandescent filaments, and cutting tools.
- Zirconium Carbonate (CAS
RN: 12671-00-0):
white
powder used
as in manufacturing of
zirconium compounds, manufacturing paint drier auxiliaries,
pigments, various
catalysts and paper sizings.
- Zirconium Dicarbide (ZrC2,
CAS RN: 12340-54-4)
- Zirconium Fluoride (ZrF4,
CAS RN: 7783-64-41,
3569-28-3,
13814-22-7,
13842-94-9)
- Zirconium Hydride (ZrH2,
CAS RN: 7704-99-6, 13940-37-9): flammable,
gray-black powder; used in powder metallurgy and nuclear moderators, and as a
reducing agent, vacuum-tube getter, and metal-foaming agent.
- Zirconium Hydroxide (Zr(OH)4 CAS
RN:12688-15-2,
14475-63-9):
toxic, amorphous white powder; insoluble
in water, soluble in dilute mineral acids; decomposes at 550°C; used in
pigments, glass, and dyes, and in making other zirconium compounds.
- Zirconium Iodide (ZrI2,
CAS RN: 14728-76-8,
13779-87-8,
15513-85-6)
- Zirconium Nitrate (CAS
RN: 12372-57-5)
- Zirconium Nitride (ZrN,
CAS RN: 25658-42-8): hard, brassy powder that is
soluble in concentrated acids; melts at 2930°C; used in refractories, cermets,
and laboratory crucibles.
- Zirconium Oxychloride (ZrOCl2,
CAS RN: 7699-43-6): White crystals that are soluble in water, insoluble in organic solvents, and
acidic in aqueous solution; used for the manufacture of other zirconium salts, textile dyeing,
oil-field acidizing, in
cosmetics and greases, and for antiperspirants and water repellents and TIO2 pigment coating.
- Zirconium Oxynitrate (ZrO(NO3)2,
CAS RN:13826-66-9)
- Zirconium Phosphate (ZrO(H2PO4)2):
toxic, dense
white powder insoluble in water, soluble in acids and organic solvents;
decomposes on heating; used as an analytical reagent, coagulant, and
radioactive-phosphor carrier.
- Zirconium Silicate
(ZrO4Si,
CAS RN: 10101-52-7)
- Zirconium silicide (CAS
RN: 12039-90-6)
- Zirconium sulfide (CAS
RN: 12039-15-5)
- Zirconium Tetrachloride (ZrCl4,
CAS RN: 10026-11-6): toxic, white lustrous monoclinic crystals;
decomposes in water, soluble in alcohol,
sublimes above 300°C; used to
make pure zirconium and for water-repellent textiles.
- Zirconium-95: radioactive isotope of zirconium
(Half-life
of 63 days with beta and gamma radiation); used as a catalyst in a cracking plant
and to trace petroleum circulation flows.
- Zirkelite A black
mineral consisting of an oxide of zirconium, titanium, calcium, ferrous iron,
thorium, uranium, and rare earths.
- Calcium Zirconate (CaZrO3 CAS
RN: 12013-47-7)
|
| SALES
SPECIFICATION |
|
APPEARANCE
|
gray-black powder
|
| CONTENT |
95.5% min
|
|
HYDROGEN
|
1.85
- 2.1% |
| TRANSPORTATION |
| PACKING |
|
| HAZARD CLASS |
4.1 |
| UN
NO. |
1437 |
| OTHER
INFORMATION |
|
Flammable
solid
PRICE
INFORMATION

|
|